Friday, 4 March 2016
Wednesday, 2 March 2016
Work power energy
WORK POWER ENERGY
WORK
Whenever a force acting
on a body, displaces it in its direction, work is said to be done by the force.
Work done by a force is equal to scalar product of force applied and
displacement of the point of application,
|
W=F. d
|
Note : Work is a scalar
quantity
1.1 WORK DONE BY A CONSTANT FORCE
If the direction and
magnitude of a force applied on a body is constant, the force is said to be
constant. Work done by a constant force,
W = Force × component
of displacement along force
The work done will, be W =
(F cos ѳ)d
= F(d
cos ѳ)
In vector form, W=F.d
Note: The force of
gravity is the example of constant force; hence work done by it is the example
of work done by a constant force.
1.2 WORK DONE BY A VARIABLE
FORCE
If the force applied on
a body is changing its direction or magnitude or both, the force is said to be
variable. Suppose a variable force causes displacement in a body from position
to position
To calculate the work done by the force
the path from
to
can
be divided into infinitesimal elements, each element is so small that during
displacement of body through it, the force is supposed to be constant. If dr̅ be small displacement of point of application and
F̅ be the force applied on the body, the work
done by force is
dW=F̅.dr̅
The total work done in
displacing body from
to
is given by
1.4 NATURE OF WORK DONE→
(1) POSITIVE WORK
W=Fs cosѳ
If the angle Ѳ is acute (Ѳ < 90°) then the work is said to be
positive. The positive work signifies that the external force favours the
motion of the body.
.
When
a body falls freely under the action of gravity (Ѳ =0°), the work done by
gravity is positive.
.
When a spring is stretched, stretching force and the displacement both are in
the same direction. So work done by stretching force is positive.
(2) Negative
work
W=Fs cosѳ
If the angle Ѳ is
obtuse (Ѳ > 90°).
Then the work is said
to be negative.
It signifies that the
direction of force is such that it opposes the motion of the body.
.
Work done by frictional force is negative when it opposes the motion.
.
Work done by braking force on the car is negative.
Wednesday, 24 February 2016
Semiconductor "Basic Electronics"
Semiconductor
- Materials that permit flow of electrons are called conductors (e.g., gold, silver, copper, etc.).
- Materials that block flow of electrons are called insulators (e.g., rubber, glass, Teflon, mica, etc.).
- Materials whose conductivity falls between those of conductors and insulators are called semiconductors.
- Semiconductors are “part-time” conductors whose conductivity can be controlled. Silicon is the most common material used to build semiconductor devices
Special points about Silicon
- Atoms in a pure silicon wafer contains four electrons in outer orbit (called valence electrons).
- Germanium is another semiconductor material with four valence electrons.
- In the crystalline lattice structure of Si, the valence electrons of every Si atom are locked up in covalent bonds with the valence electrons of four neighboring Si atoms.
- In pure form, Si wafer does not contain any free charge carriers.
- An applied voltage across pure Si wafer does not yield electron flow through the wafer.
- A pure Si wafer is said to act as an insulator.
- In order to make useful semiconductor devices, materials such as phosphorus (P) and boron (B) are added to Si to change Si’s conductivity.
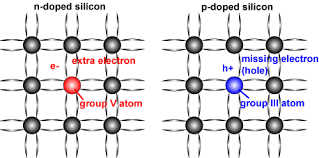
N-Type Silicon
- Pentavalent impurities such as phosphorus, arsenic, antimony, and bismuth have 5 valence electrons.
- When phosphorus impurity is added to Si, every phosphorus atom’s four valence electrons are locked up in covalent bond with valence electrons of four neighboring Si atoms. However, the 5th valence electron of phosphorus atom does not find a binding electron and thus remains free to float. When a voltage is applied across the silicon-phosphorus mixture, free electrons migrate toward the positive voltage end.
- When phosphorus is added to Si to yield the above effect, we say that Si is doped with phosphorus. The resulting mixture is called N-type silicon (N: negative charge carrier silicon).
- The pentavalent impurities are referred to as donor impurities.
P-Type Silicon
- Trivalent impurities e.g., boron, aluminum, indium, and gallium have 3 valence electrons.
- When boron is added to Si, every boron atom’s three valence electrons are locked up in covalent bond with valence electrons of three neighboring Si atoms. However, a vacant spot “hole” is created within the covalent bond between one boron atom and a neighboring Si atom. The holes are considered to be positive charge carriers.
- When a voltage is applied across the silicon-boron mixture, a hole moves toward the negative voltage end while a neighboring electron fills in its place.
- When boron is added to Si to yield the above effect, we say that Si is doped with boron. The resulting mixture is called P-type silicon (P: positive charge carrier silicon).
- The trivalent impurities are referred to as acceptor impurities.
Bulk Modulus Elasticity
The Bulk Modulus Elasticity is a material property characterizing the compressibility of a fluid - how easy a unit volume of a fluid can be changed when changing the pressure working upon it.
The Bulk Modulus Elasticity can be expressed as
E = - dp / (dV / V) .....................(1)whereE = bulk modulus elasticitydp = differential change in pressure on the objectdV = differential change in volume of the objectV = initial volume of the object
The Bulk Modulus Elasticity can alternatively be expressed as
E = dp / (dρ / ρ) ..........................(2)wheredρ = differential change in density of the objectρ = initial density of the object
An increase in the pressure will decrease the volume ...............(1).
A decrease in the volume will increase the density.................. (2).
- The SI unit of the bulk modulus elasticity is N/m2 (Pa)
- The imperial (BG) unit is lbf/in2 (psi)
- 1 lbf/in2 (psi) = 6.894 103 N/m2 (Pa)
****A large Bulk Modulus indicates a relative in compressible fluid.
Tuesday, 23 February 2016
SURFACE TENSION
Surface Tension
“Surface tension is the property of a liquid by virtue of which its free surface behaves like a stretched membrane and supports, comparatively heavier objects placed over it”. It is measured in terms of force of surface tension.The free surface of a liquid contracts so that its exposed surface area is a minimum, i.e., it behaves as if it were under tension, somewhat like a stretched elastic membrane. This property is known as surface tension. The surface tension of a liquid varies with temperature as well as dissolved impurities, etc. When soap is mixed with water, the surface tension of water decreases. Also, the surface tension decreases with increase in temperature.
- Force of cohesion:- It is force between two molecules of similar nature.
- Force of adhesion:- It is the force between two molecules of different nature.
- Molecular range:- The maximum distance between two molecules so that the force of attraction between them remains effective is called molecular range.
- Sphere of influence:- Sphere of influence of any molecule is the sphere with molecule as its center and having a radius equal to molecular range (=10-7 cm).
- Surface film:- Surface film of a liquid is defined as the portion of liquid lying on the surface and caught between two parallel planes situated molecular range apart.
- Surface tension:-
Surface tension is the property of a liquid by virtue of which its free surface behaves like a stretched membrane and supports, comparatively heavier objects placed over it. It is measured in terms of force of surface tension.
- Force of surface tension:- It is defined as the amount of force acting per unit length on either side of an imaginary line drawn over the liquid surface.
(a) T = Force/length = F/l
(b) T = Surface energy/Surface area = W/A
Units:- S.I – Nm-1
C.G.S- dyn cm-1
Properties of Surface Tension:-
- Scalar quantity.
- Temperature sensitive.
- Impurity sensitive.
- Depends only n the nature of the liquid.
- Unit of surface tension, N/m.
- Dimension of surface tension, ML0T-2.
How do detergents clean dirty clothes?
Consider a wire frame (see the adjacent figure) equipped with a sliding wire AB. It is dipped in a soapy water. A film of liquid is formed on it. A force F has to be applied to hold the wire in place. Since the soap film has two surfaces attached to the wire, the total length of the film in contact with the wire is 2L.T (surface tension) = F/2L.
Surface tension of a liquid is measured by the normal force acting per unit length. On either side of an imaginary line drawn on the free surface of a liquid, the direction of this force is perpendicular to the line and tangential to the free surface of liquid.
Wednesday, 17 February 2016
Simple Harmonic Motion
Simple harmonic motion is typified by the motion of a mass on a spring when it is subject to the linear elastic restoring force given by Hooke's Law. The motion is sinusoidal in time and demonstrates a single resonant frequency.
Mass on Spring: Motion Sequence
A mass on a spring will trace out a sinusoidal pattern as a function of time, as will any object vibrating in simple harmonic motion. One way to visualize this pattern is to walk in a straight line at constant speed while carrying the vibrating mass. Then the mass will trace out a sinusoidal path in space as well as time.
Concept of periodic motion
Monday, 15 February 2016
UNITS & DIMENSIONS
Fundamental concepts of the Physics start from this chapter. Basically the terms & concepts which are illustrated in this topic will be used in so many ways because all Physical quantities have units. It is must to measure all Physical quantities so that we can use them. In this topic we will have an over view of different units of different Physical quantities. We will learn the dimension and dependence of the unit of any Physical quantity on fundamental quantities or unit.
1. PHYSICAL QUANTITIES
The quantities by means of which we describe the laws of physics are called physical quantities.
There are two type of physical quantities.
1.1 Fundamental quantities
1.2 Derived quantities
1.1 Fundamental quantities
Physical quantities which are independent of each other and cannot be further resolved into any other physical quantity are known as fundamental quantities. There are seven fundamental quantities.
Fundamental quantity Units Symbol
(a) Length Metre m
(b) Mass Kilogram kg
(c) Time Second s
(d) Electric current Ampere A
(e) Temperature Kelvin K
(f) Luminous intensity Candela Cd
(g) Amount of substance Mole Mol.
1.2 Derived Quantities :
Physical quantities which depend upon fundamental quantities or which can be derived from fundamental quantities are known as derived quantities.
Derived Physical Quantities:
| S.No | Derived Physical Quantity | Formula | Dimensional Formula | S.I Unit of physical quantity |
| 1. | Area | [ | ||
| 2. | Volume | [ | ||
| 3. | Density | [ | ||
| 4. | Specific Gravity | [ | No units | |
| 5. | Frequency | [ | hertz | |
| 6. | Angle | No units | ||
| 7. | Velocity | m/sec | ||
| 8. | Speed | m/sec | ||
| 9. | Areal velocity | |||
| 10. | Acceleration | |||
| 11. | Linear momentum | kg m/sec | ||
| 12. | Force | kg-m/ | ||
| 13. | Weight | w=mg | kg-m/ | |
| 14. | Moment of force/Torque/Couple | kg | ||
| 15. | Impulse | kg m/sec or Ns | ||
| 16. | Pressure | N/ | ||
| 17. | Work | Nm or Joule | ||
| 18. | Kinetic Energy | joule | ||
| 19. | Potential Energy | mgh | joule | |
| 20. | Gravitational constant | |||
| 21. | Gravitational field strength | |||
| 22. | Gravitational Potential | |||
| 23. | Force constant (k) | |||
| 24. | Power | W or J/sec | ||
| 25. | Moment of Inertia ( I ) | kg | ||
| 26. | Stress | N/ | ||
| 27. | Strain | No units | ||
| 28. | Modulus of Elasticity | N/ | ||
| 29. | Poission’s Ratio | σ = | No units | |
| 30. | Velocity gradient | |||
| 31. | Coefficient of dynamic viscosity | kg | ||
| 32. | Surface Tension | |||
| 33. | Angular displacement ( | no Units | ||
| 34. | Angular velocity(ω) | rad/sec | ||
| 35. | Angular acceleration(α) | rad/ | ||
| 36. | Angular momentum | Iω | ||
| 37. | Angular Impulse | Iω | ||
| 38. | Temperature | kelvin or degree Celsius | ||
| 39. | Coefficient of linear expansion(α) | /kelvin | ||
| 40. | Specific heat | |||
| 41. | Latent heat | |||
| 42. | Entropy | |||
| 43. | Thermal capacity | |||
| 44. | Gas constant | |||
| 45. | coefficient of thermal conductivity | |||
| 46. | Pole strength | Am | ||
| 47. | Magnetic Moment | |||
| 48. | Magnetic flux | weber ; | ||
| 49. | Magnetic field,magnetic flux density (B) | Tesla; | ||
| 50. | Permeability of free space | |||
| 51. | Magnetic susceptibilty also called volumetric or bulk susceptibility χm | χm = μr − 1 | no units | |
| 52. | Electric Charge | Amp sec , coul | ||
| 53. | Electric potential | Volt | ||
| 54. | E.M.F | Volt | ||
| 55. | Electric Capacity | Farad | ||
| 56. | Electric Resistance | Ohm (Ω) or volt/amp | ||
| 57. | Resistivity | Ohm mt (Ω-m) | ||
| 58. | Conductivity | 1/ | Siemens/m | |
| 59. | Permittivity |
| farad/m | |
| 60. | Electric conductance | Siemens (or) mhos | ||
| 61. | Electric power | Watt | ||
| 62. | Electrical Impedance(Z) | Ohm (Ω) or volt/amp | ||
| 63. | Electrical admittance | 1/Z(Reciprocal of electric impedance) | Siemens (or) mhos | |
| 64. | Self Inductance(L) | weber/amp or Henry | ||
| 65. | Boltzmann’s constant | J/kelvin | ||
| 66. | Stefan’s constant | |||
| 67. | Co-efficient of friction | dimension less scalar | no units | |
| 68. | Dielectric constant | It is also called relative permittivity | dimension less | no units |
| 69. | Planck’s constant | J.sec (or) eV.sec | ||
| 70. | Refractive index | μ | no units | |
| 71. | Focal length(f) | Distance between center of the lens(mirror) to its focus | L | meter |
| 72. | Power of a lens (P) | The reciprocal of the focal length of a lens in meters is called power of a lens; p=1/f | diaptors | |
| 73. | Wave number | No.of waves/distance | ||
| 74. | Wave length | Length of a wave | L | meter |
2. UNITS
Definition : Things in which quantity is measured are known as units.
Measurement of physical quantity = (Magnitude) × (Unit)
Ex.1 A physical quantity is measured and the result is expressed as nu where u is the unit used
and n is the numerical value. If the result is expressed in various units then :
(A) n ∝ size of u
(B) n ∝ u2
(C) n ∝ u
(D) n ∝ u
1
Answer : (D)
There are three types of units
2.1 Fundamental or base units
2.2 Derived units
2.3 Supplementary units
2.1 Fundamental or base units:
Units of fundamental quantities are called fundamental units.
2.1.1 Characteristics of fundamental units:
(i) they are well defined and are of a suitable size
(ii) they are easily reproducible at all places
(iii) they do not vary with temperature, time pressure etc. i.e. invariable.
(iv) there are seven fundamental units.
2.1.2 Definitions of fundamental units:
1 Metre :
The distance travelled by light in Vacuum in 1 second is called 1m.
2 Kilogram :
The mass of a cylinder made of platinum iridium alloy kept at international bureau of weights and measures is defined as 1kg.
3 Second :
Cesium -133 atom emits electromagnetic radiation of several wavelengths. A particular radiation is selected which corresponds to the transistion between the two hyperfine levels of the ground state of Cs - 133. Each radiation has a time period of repetition of certain characteristics. The time duration in 9, 192, 631, 770 time periods of the selected transistion is defined as 1s.
4 Ampere :
Suppose two long straight wires with negligible cross-section are placed parallel to each other in vacuum at a seperation of 1m and electric currents are established in the two in same direction. The wires attract each other. If equal currents are maintained in the two wires so that the force between them is 0.0000002 newton per meter of the wire, then the current in any of the wires is called 1A. Here, newton is the SI unit of force.
5 Kelvin :
The fraction 1/273.16 of the thermodynamic temperature of triple point of water is called 1K.
6 Mole :
The amount of a substance that contains as many elementary entities (Molecules or atoms if the substance is monoatomic) as there are number of atoms in .012 kg of carbon - 12 is called a mole. This number (number of atoms in 0.012 kg of carbon-12) is called Avogadro constant .
7 Candela:
The S.I. unit of luminous intensity is 1cd which is the luminous intensity of a blackbody of surface area 1/600,000 metre square placed at the temperature of freezing platinum and at a pressure of 101,325 newton per meter square, in the direction perpendicular to its surface.
Different quantities with units. symbol and dimensional formula,
Subscribe to:
Posts (Atom)